The Mertz Glacier as seen in 2007, extending 75 km out into the Southern Ocean
Antarctica was in the news this weekend when a 97 kilometer long iceberg the size of Luxembourg collided with the floating Mertz Glacier, breaking the famous glacier off at the base and generating a 2500 sq. kilometer iceberg. Each of these behemoths weigh several hundred billion tons, so the impact must have been quite a crunch!

Iceberg B9B collides with Mertz Glacier Tongue
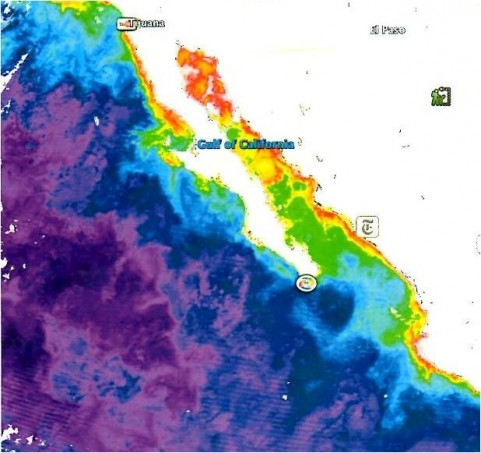
At right is an image taken February 20th, several days after the impact: the broken Mertz Glacier Tongue is on the left side of the photo, and the colliding B9B iceberg is near the center-right. The Mertz Glacier, which was sheared off at the base, was a significant barrier to westward drifting sea ice. The Mertz Glacier is on the George V coast of East Antarctica, a region is famous for its high-velocity katabatic winds: sustained wind velocities at nearby Dumont D’Urville have reached 199 m.p.h! These winds blow the pack ice out to sea, and because of the blocking geometry of the Mertz Glacier, this area generally remains ice-free all winter.
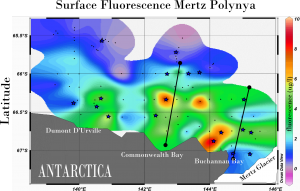
Fluorescence map of the Mertz Polynya in December 2007 (mertz Glacier is in lower right). Surface blooms are in red, and marine metagenomic samples were taken in areas marked with a star.
In the Austral summer of 2007, scientists from the J. Craig Venter Institute visited this ice-free area, or polynya, as part of the International Polar Year’s Census of Antarctic Marine Life (CAML). Because sunlight can freely penetrate the water column, polynyas are areas of enhanced productivity. Diatoms and other phytoplankton form massive springtime blooms, supporting whales, penguins, and much of the Antarctic food chain. Above is a fluorescence ‘bloom map’ of the Mertz Polynya, just west of the Mertz Glacier. Our expedition on board the Aurora Australis attempted to capture a biological snapshot of the entire region, and Jeff Hoffman and I were able to collect samples ranging from thick blooms of Phaeocystis antarctica to oligotrophic cold-water upwellings at the base of the Mertz Glacier.

CTD Rosette being deployed at the base of the Mertz Glacier to collect a sample from 1320m in depth
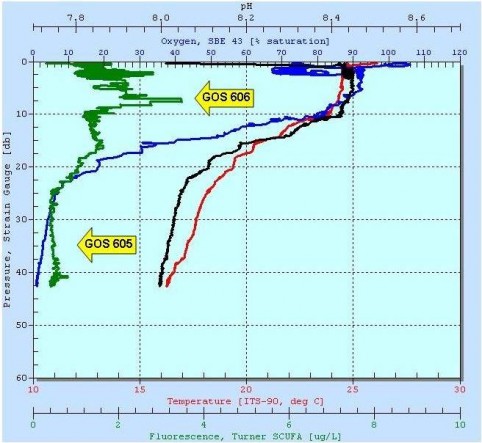
The region around the Mertz Glacier is equally famous as one of three regions where Antarctic Bottom Water is formed (the other two are the Ross and Weddell Seas). Bottom water is created where saline water is extruded from newly formed sea ice. This cold dense water sinks from the surface and becomes distributed into all of the world’s major ocean basins. Because the sea-ice in a polynya is continuously formed and blown out to sea, there is near continual production of brine and bottom water. While in the Mertz Ploynya, Jeff and I used the ships 24-bottle CTD rosette to sample some of this bottom water, and one of the samples came from Buchanan Bay, right next to the area where the glacier split. This sample came from a depth of 1320m, and may yield insight into bacterial activities at the base of the water column. Additional deep water samples were taken in the Adelie Depression , the Mertz Bank, and the Mertz Depression, and one sample came from a depth of 3,690 m in the Southern Ocean.
Almost half of the water samples we collected have been sequenced using 454 sequencing technology and are in the process of annotation. This biological data will form an important baseline as this region undergoes rapid change: loss of the protective geometry of the Mertz Glacier will likely cause changes in the formation of the Mertz Polynya, influencing both the biology of the annual spring bloom and the dynamics of bottom water formation. Stay tuned for more updates on this exciting event and on the microbiology of the region.

Tafelbergs floating in the morning light, Mertz Polynya, December 2007